Letter to the Investors
Komipharm is developing a therapeutic treatment for the infectious disease, COVID-19.
1. Characteristics of COVID-19 (including variants of the SARS-CoV-2 virus)
A severe acute respiratory disease named ‘coronavirus disease 2019’ (COVID-19) has caused a global pandemic and the recent emergence of the delta variant of SAR-CoV-2 is adding to the growing anxiety.
Viruses are not living organisms and they take over the normal host cells to replicate, and COVID-19 extends its reaches to many organs including the lungs. Vaccination has been considered as the only approach to treat virus-caused diseases as complete elimination of viruses will inevitably damage the host cell.
Due to the frequent emergence of mutated variants of the original virus, and the long, complex process of vaccine development, extra efforts were made to combat the outbreak by using repurposed drugs for the treatment of COVID-19. The World Health Organization (WHO) performed a study involving 11,266 patients from 405 hospitals of 30 countries, looking at the effects of remdesivir, hydroxychloroquine, lopinavir/ritonavir and interferon regimens on overall mortality, initiation of ventilation, and duration of hospital stay in hospitalized COVID-19 patients. The study concluded that these regimens had little or no effect on 28-day mortality or the in-hospital course of COVID-19 among hospitalized patients ( https://pubmed.ncbi.nlm.nih.gov/33264556 ). These tested drugs were suggested to have an antiviral activity in the body, however they failed to stop the replication of COVID-19 viruses in the cell.
2. Antiviral mechanisms of action of PAX-1
How can we deliver the antiviral drug into the cell to eliminate viruses, and how can we allow the drug to specifically target the COVID-19 virus while maintaining the cellular function?
The best antiviral treatment should not involve the complete elimination of viruses, but rather aim to block the viral replication.
PAX-1, an anti-cancer drug, has the mechanism of inhibiting human telomerase reverse transcriptase (hTERT), which is required for the transcription and proliferation of cancer cells. (Published 14thAugust 2008: https://pubmed.ncbi.nlm.nih.gov/18628474 )
The article reported that hTERT possesses RdRP (polymerase) activity required for oncogenesis ( https://pubmed.ncbi.nlm.nih.gov/32805774 ), which indicates that RNA viruses and cancer cells share similar survival strategies associated with RNA dependence and RNA synthesis.
PAX-1 inhibits the transcription of hTERT and interferes with RdRP activity, thereby exhibiting antiviral effects by blocking the enzyme required for viral replication. This is a very unusual mechanism.
3. Anti-inflammatory mechanism of action of PAX-1
In addition to the antiviral drug effect of PAX-1 on COVID-19, PAX-1 suppresses the activity of the transcription factor (NF-kB) in cells and suppresses the secretion of cytokine factors that cause inflammation, without being toxic to the normal inflammatory cells. This mechanism leads to reduction of virus-caused inflammation by limiting the release of the proinflammatory mediators such as TNF-α, IL-6, IL-1β, iNOS and COX-2, which is the key to the treatment of inflammation.
4. Animal experiment with PAX-1 showed antiviral and anti-inflammatory efficacy of PAX-1 on COVID-19
A subculture of SARS-CoV-2 (NCCP 43326) was prepared in PBS solution and 200 μL of the preparation was injected into the left nasal cavity of Syrian hamsters. The infected hamsters were divided into two groups where animals were either untreated or treated with PAX-1. The dose of PAX-1 used for this study (0.9 mg/kg) was equivalent to human dose of 7.5 mg/day. On the third day of post-infection, treatment with PAX-1 for 2 days resulted in significant reduction of the copy number of SARS-CoV-2 gene compared to the untreated group, indicating a reduced viral replication.
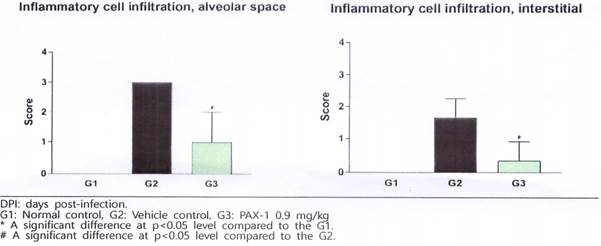
In addition, the level of inflammation in the lungs from virus-infected animals was examined in the PAX-1 treatment group, where the level of infiltration of inflammatory cells in the alveolar cavity of the lungs was significantly reduced in the drug treated group compared to the untreated control group.
Significant weight loss is one of the most prevalent clinical symptoms observed from COVID-19 patients. The body weight of the virus-infected animals that received PAX-1 treatment showed normal body weight while those animals who did not receive the treatment experienced significant weight loss over the period of 7 days.
5. Safety aspects of PAX-1
Cancer patients involved in the previous clinical trials with PAX-1 for cancer treatment had taken up to 8 tablets (20mg/day) of the drug per day. And PAX-1 was recognized by the US FDA as an orphan drug for glioblastoma brain cancer treatment on the 1st of July 2021, and a phase 2 clinical trial is currently in progress in Australia and Korea.
The US FDA has approved the dosage for the treatment of glioblastoma for up to 6 tablets per day (15 mg/day). For studies related to COVID-19 treatment, we expect to find a safe and therapeutic effect from taking 2 to 3 tablets per day in COVID-19 patients, based on the safety/efficacy results from preclinical and existing clinical trials.
6. Future direction of PAX-1 development
As explained above, we have confirmed the inhibitory effect of PAX-1 on viral replication and inflammation.
Based on this, our primary focus is placed on the development of PAX-1 as a therapeutic drug for respiratory patients infected with COVID-19, followed by the development of PAX-1 as a preventive treatment for COVID-19.
The mutated viruses emerged from the COVID-19 virus are also RNA viruses, and we expect to see the same mechanism of action of PAX-1 in the variants. Clinical trial is under development in countries where mutated virus such as delta mutant virus has emerged.
We adhere to the WHO ICH guideline for the development of PAX-1 as a treatment for COVID-19.
As our shareholders know, we have applied for an international patent for PAX-1’s reduction of an inflammatory response due to a viral infection and a method of treating or preventing an inflammatory condition due to a viral infection last year.
The purpose of today's letter to the shareholders is to inform about our first stage of PAX-1 development as a treatment of COVID-19.
The second and third stages of development will be shared as the development unfolds.
We ask for your support from shareholders for successful development.
7. Treatment with PAX-1 inhibits replication of virus and reduces inflammation in COVID-19 infected hamsters
Real time qRT-PCR (virus number comparison table)
Komipharm International Co Ltd
-
- 이전글
- 주주님께 드리는 글 (COVID-19 미얀마 정부 연구자임상시험 개시)
- 21.10.05
-
- 다음글
- 주주님께 드리는 글(COVID-19 감염 대상 치료제 개발진행 1단계설명)
- 21.07.21